In four months the Food and Drug Administration went from investigating whether e-cigarettes motivated teens to smoke to declaring teen vaping an “epidemic” where “all options are on the table” to prevent “addicting a generation of youth on nicotine.” What followed has been described as a “historic crackdown” of the e-cigarette industry.
This crackdown included fining over 1,300 retailers for allegedly selling vaping goods to minors, demanding five vaping companies provide the FDA “with robust plans on how they’ll convincingly address the widespread use of their products by minors,” and seizing records from e-cigarette industry leader Juul’s headquarters. The FDA has also proposed a list of heavy-handed regulations, including banning flavored e-juices, banning convenience stores from selling e-cigarettes, and requiring age-verification for online e-cigarette sales. Although these regulations are still in the proposal phase, many producers are preparing for dramatic changes to the e-cigarette market.
I’ve argued in a previous blog post that these regulations will likely cause considerable harm to those they are intended to help. Others agree. However, few have asked, Can the FDA actually prevent teens from vaping?
I am, once again, skeptical.
Those calling for more oversight and regulation of the e-cigarette market seem to have overlooked that the market was regulated well before recent FDA efforts. In 2014, forty states had banned e-cigarette sales to minors. The FDA first passed regulation banning e-cigarette sales to minors in 2016. But the rate of underage e-cigarette use has increased since 2016, meaning previous regulations did not affect teen vaping and might have made the issue worse. Further, teen vaping rates were comparatively lower when states determined vaping laws.
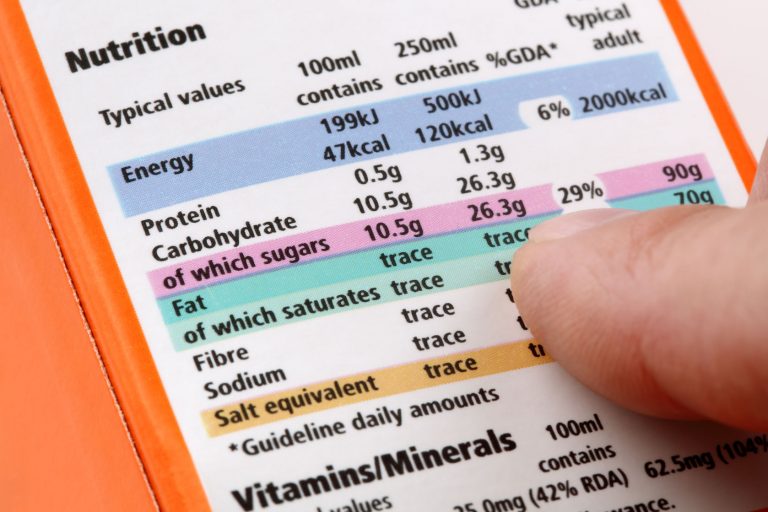
The FDA’s efforts to mitigate obesity (also considered an epidemic) have also fared poorly. Beginning in 2004, the FDA launched an anti-obesity campaign that included requiring more detailed nutritional labeling and allowing low-calorie foods more freedom to advertise health claims. Since 2004, obesity rates have increased considerably, and a growing number of consumers feel tricked or confused by nutritional labels.
When we consider the FDA’s persistent history of gaining more influence while failing to achieve its objectives as well as its recent failures, we should be concerned when it gains “evolving regulatory powers” into e-cigarettes and other products. Epidemics are causes for concern, but before we declare regulation to be the cure, we should question who administers the treatment.